particulate nature of matter
- Matter is everything that occupies space and has mass
- The particle theory matter states that the matter is made up of a large number of tiny and discrete particles .
Matter is made up of a large number of tiny and discrete particles
Type of particles
- Particles can exist as atoms , molecules or ions
- Atom is the smallest , indivisible particle of an element .
- Molecules are particles that made up of two or more atom
- Ion are particles that carry charge .
b) negative ion - anion
RECOMMENDED VIDEOS
SPM CHEMISTRY - PARTICLE THEORY OF MATTER
INTRODUCTION TO PARTICLES THEORY OF MATTER LECTURE
ATOMIC THEORY
Characteristics of Matter in Solid, Liquid and Gaseous State
| Characteristics |
Solid
|
Liquid
|
Gas
|
 |  |  | |
Arrangement of Particles
|
Particles are arranged inorderly manner and close to one another.
|
Particles are not arranged in order. The space between particles is moderately large.
|
The particles are very far apart and randomly arrange.
|
Movement of Particles
|
Particles vibrate at fixed positions.
|
Particles move randomly and slowly and sometimes will collide against each other.
|
The particles move randomly in all directions at great speed.
|
Force of Attraction between particles
|
very strong
|
Strong but weaker than in the solid state.
|
very weak
|
Ability to be compressed
|
Very difficult to be compressed because the particles are packed closely.
|
Not easily compressed because the particles are packed quite closely.
|
Easily compressed because the particles are very far apart.
|
Volume
|
Fixed
|
Fixed
|
Follows the container
|
Heat Energy content
|
Lowest Energy Content
|
Moderate energy content.
|
Highest
energy content
|
Shape
|
Fixed
|
Follows the container
|
Fills the whole container
|
Matter can divided into element and compound
ELEMENTS
EXAMPLES :
(Both the iron and oxygen are element because they consist of only one type of atoms)
COMPOUNDS
- A compound is a substance composed of molecules made up of atoms of two or more elements.
- A compound is made up of either molecules or ions
(Both the sodium chloride and carbon dioxide are compound because they consist of more than one type of atoms)
Change in Heat and Kinetic Energy of Particles
- The change in temperature will influences the kinetic energy or the speed of the motion of the particles.
- When a substance is heated, the kinetic energy of the particles in the substance increases. This causes the particles to move or vibrate faster.
- Likewise, when a substance is cooled, the kinetic energy of the particles in the substance decreases. This causes the particles to move or vibrate slower.
- The kinetic energy of the particles in a substance is directly proportional to the temperature of the substance.
Melting
| Definition Melting is the process where a solid changes to its liquid state at a certain temperature (called the melting point) and pressure when it is heated.
Notes
|
Freezing
| Definition Freezing is the process where a liquid changes to its solid state at a certain temperature (called freezing point) and pressure when it is cooled. Notes
|
Vaporizaon
(Evaporation)
| Definition Vaporization, also called evaporation is the process whereby atoms or molecules in a liquid state gain sufficient energy to enter the gaseous state. Boiling is the rapid vaporization of a liquid at a certain temperature (the boiling point) and pressure when heat is applied to it. Notes Evaporation
|
Condensation
| Definition Condensation is the process by which a gas or vapor changes to liquid state at certain temperature and pressure when it is cooled. Notes
|
sublimation
| Defintion Sublimation is a process of conversion of a substance from the solid to the vapour state without its becoming liquid. Notes
|
Explanation on the heating process
A
|
|
A-B
|
|
B
|
|
B-C
|
|
C
|
|
C-D
|
|
D
|
|
D-E
|
|
E
|
|
E-F
|
|
Recommended video
How to read heating curve
Explanation on the cooling process
The graph above shows the cooling curve of a substance.
P
|
|
P-Q
|
|
Q
|
|
Q-R
|
|
R
|
|
R-S
|
|
S
|
|
S-T
|
|
T
|
|
T-U
|
|
U
|
|
Recommended video
Cooling Process
Melting Point, Boiling Point and States of Matter
How to read cooling curve
B.THE ATOMIC STRUCTURE
History Of Development Of The Model Of Atom
Table below shows the scientists that contribute to the development of the Model of Atom.
John Dalton
 |
|
J.J. Thomson
 |
|
Ernest Rutherford
 |
|
Neils Bohr
 |
|
James Chadwick
 |
|
Recommended Videos
Models of the Atom Timeline
Models of the Atom: Thompson, Rutherford and Bohr Models
Thomson's Plum Pudding Model of the Atom
Modern Atomic Model
According to the modern atomic model,
- The central nucleus consists of protons and neutrons. It containing almost all the mass of the atom.
- The nucleus of an atom is very small compared to the size of the atom
- The electrons are orbiting outside the nucleus in the electron shells
- The electrons are moving in electron shells at a very high speed and we cannot determine the position of the electrons at a particular time
The Subatomic Particles Of An Atom
- Atoms are made up of tiny particles called subatomic particles.
- An atom contains three types of subatomic particles:
- proton,
- neutron and
- electron,
- The proton and neutron form the nucleus at the centre of an atom. They are also called the nucleon of an atom.
- The electron moves around the nucleus at a very high speed.
- The nucleus is positively charged because of the presence of protons, which are positively charged. The neutrons are neutral.
- The symbols, charge and relative masses of proton, neutron and electron are as below.
| Particle | Symbol | Relative charge | Relative mass |
| Proton |
p
|
+1
|
1
|
| Neutron |
n
|
0
|
1
|
| Electron |
e
|
-1
|
1/1840
|
Proton Number And Nucleon Number
- Proton number = the number of protons
- Nucleon number = Number of protons + Number of neutrons
Proton Number
- The proton number (Z) represent the number of protons found in the nucleus of an atom.
- Proton number = the number of protons
- The proton number is also known as the atomic number.
- In an atom of neutral charge, the number of electrons also equals the atomic number.
- Hence, the proton number of an atom can also represent the number of electrons.
Nucleon Number
- The nucleon number (A), also called atomic mass number or mass number, is the number of protons plus the number of neutrons in an atomic nucleus. (Nucleon number = Number of protons + Number of neutrons)
- The nucleon number of an atom is about the same as the mass of the atom because the mass of an electron is very small and can be ignored.
Atom
|
Proton Number
|
Nucleon Number
|
Amount of Proton
|
Amount of electron
|
Amount of Neutron
|
| Helium |
2
|
4
|
2
|
2
|
2
|
| Oxygen |
8
|
16
|
8
|
8
|
8
|
| Sodium |
11
|
23
|
11
|
11
|
12
|
| Chlorine |
17
|
35
|
17
|
17
|
18
|
[Notes: In ion, the amount of protons IS NOT equal to the amount of electrons]
The Charge Of Particles
- A neutral atom contains the same number of electrons as the protons.
- The positive and negative charges of the protons and electrons respectively neutralise each other, for example, (+4) + (-4) = 0
- If the number of protons is greater than the number of electron, the particle is positively charge.
- If the number of protons is greater than the number of electron, the particle is positively charge.
| Number of proton | Number of electron |
Charge
|
3
|
3
|
0
|
5
|
2
|
+3
|
9
|
10
|
-1
|
11
|
10
|
+3
|
16
|
18
|
-2
|
17
|
18
|
-1
|
20
|
18
|
+3
|
C.Isotopes
Isotopes are atoms of certain elements which have the same number of protons but different number of neutrons in the nucleus of the atoms.
It can also be defined as atoms of certain elements with the same proton numbers but with different nucleon numbers.
Properties of Isotope
| |
| Number of proton | equal |
| Number of neutron | difference |
| Chemical properties | same |
| Physical properties | difference |
| Element | Name | Symbol | Proton Number | Nucleon Number | Number of proton | Number of neutron |
| Hydrogen | Hydrogen |
11H
|
1
|
1
|
1
|
0
|
| Deuterium |
21H
|
1
|
12
|
1
|
1
| |
| Tritium |
31H
|
1
|
23
|
1
|
2
| |
| Oxygen | Oxygen-16 |
168O
|
8
|
16
|
8
|
8
|
| Oxygen-17 |
178O
|
8
|
17
|
8
|
9
| |
| Oxygen-18 |
188O
|
8
|
18
|
8
|
10
| |
| Carbon | Carbon-12 |
126C
|
6
|
12
|
6
|
6
|
| Carbon-13 |
136C
|
6
|
13
|
6
|
7
| |
| Carbon-14 |
146C
|
6
|
14
|
6
|
8
| |
| Chlorine | Chlorine-35 |
3517Cl
|
17
|
35
|
17
|
18
|
| Chlorine-37 |
3717Cl
|
17
|
37
|
17
|
20
| |
| Sodium | Sodium-23 |
2311Na
|
11
|
23
|
11
|
12
|
| Sodium-24 |
2411Na
|
11
|
24
|
11
|
13
|
D.THE ELECTRONIC STRUCTURE OF AN ATOM
Electron Arrangement In Atom
- We have learnt that electrons occupy orbits with definite energy level of an atom, as suggested by Neils Bohr.
- These orbits with definite energy level are known as the shell.
- Every single shell is capable of holding up to certain amount of electrons.
- The first shell can hold up to two electrons. This is called a duplet.
- The second shell can hold up to eight electrons. This is called an octet.
- The third shell can hold up to eighteen electrons.
- However, with the third shell, when eight electrons are present, extra stability is gained. The additional electrons go into the fourth shell before the third shell is completely filled.
- The way in which the electrons are distributed in the shells of an atom is called the electron arrangement of the atom
- The examples below show the electron arrangement of some elements:
Atom
|
Notes
|
Electrons Arrangement
| ||
|
|
2.1
| ||
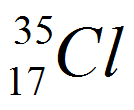 |
|
2.8.7
| ||
 |
|
2.8.8.2
|
Table below shows the arrangement of electrons of the first 20 elements in the periodic table. We shall learn periodic table in chapter 4.
| Element | Proton Number | Number of Electron |
Number of electron in
| Electron Arrangement | |||
| 1st shell | 2nd shell | 3rd shell | 4th shell | ||||
| Hydrogen |
1
|
1
|
1
|
0
|
0
|
0
|
1
|
| Helium |
2
|
2
|
2
|
0
|
0
|
0
|
2
|
| Lithium |
3
|
3
|
2
|
1
|
0
|
0
|
2.1
|
| Beryllium |
4
|
4
|
2
|
2
|
0
|
0
|
2.2
|
| Boron |
5
|
5
|
2
|
3
|
0
|
0
|
2.3
|
| Carbon |
6
|
6
|
2
|
4
|
0
|
0
|
2.4
|
| Nitrogen |
7
|
7
|
2
|
5
|
0
|
0
|
2.5
|
| Oxygen |
8
|
8
|
2
|
6
|
0
|
0
|
2.6
|
| Fluorine |
9
|
9
|
2
|
7
|
0
|
0
|
2.7
|
| Neon |
10
|
10
|
2
|
8
|
0
|
0
|
2.8
|
| Sodium |
11
|
11
|
2
|
8
|
1
|
0
|
2.8.1
|
| Magnesium |
12
|
12
|
2
|
8
|
2
|
0
|
2.8.2
|
| Aluminium |
13
|
13
|
2
|
8
|
3
|
0
|
2.8.3
|
| Silicon |
14
|
14
|
2
|
8
|
4
|
0
|
2.8.4
|
| Phosphorus |
15
|
15
|
2
|
8
|
5
|
0
|
2.8.5
|
| Sulphur |
16
|
16
|
2
|
8
|
6
|
0
|
2.8.6
|
| Chlorine |
17
|
17
|
2
|
8
|
7
|
0
|
2.8.7
|
| Argon |
18
|
18
|
2
|
8
|
8
|
0
|
2.8.8
|
| Potassium |
19
|
19
|
2
|
8
|
8
|
1
|
2.8.8.1
|
| Calcium |
20
|
20
|
2
|
8
|
8
|
2
|
2.8.8.2
|
Recommended Videos
Electron arrangement in an atom
Valence Electrons
Valence electrons are the electrons in the outermost shell.
- The electrons in the outermost shell of an atom are called valence electrons.
- The valence electrons have great significance in determining the chemical properties of an atom.
- Elements with the same number of valence electron have the same chemical properties.
Example:
Given that a sodium atom has 11 protons 12 neutrons. Find the number of valence electron in a sodium atom.
Answer:
Given that a sodium atom has 11 protons 12 neutrons. Find the number of valence electron in a sodium atom.
Answer:
For an atom,
Number of electrons = number of protons = 11
Electron arrangement of sodium = 2.8.1
Therefore, sodium has 1 valence electron.















No comments:
Post a Comment